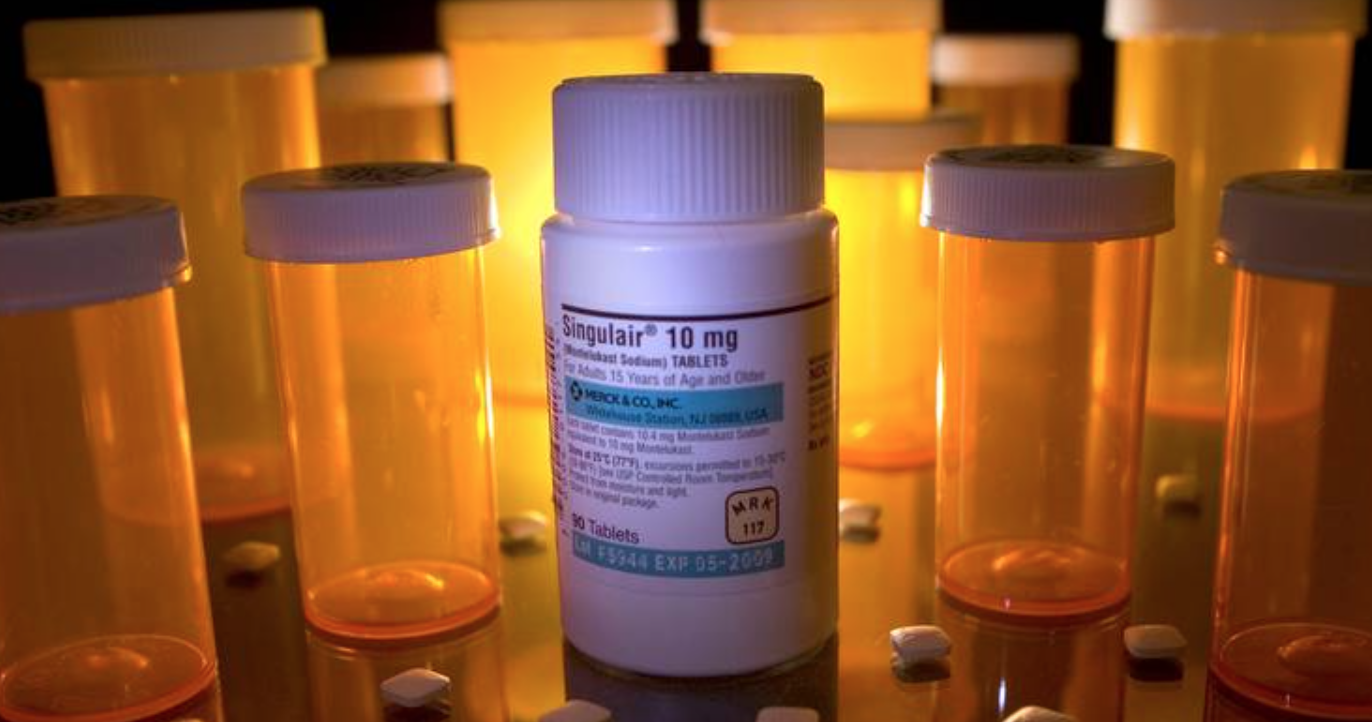
The food and drug administration (fda) announced late last week that the allergy and asthma drug montelukast, sold under the brand named singulair, will carry singulair black box warning fda a new boxed warning. fda officials. March 6, 2020 fda: asthma drug singulair to get 'black box' warning asthma and allergy drug montelukast—sold as a generic and under the brand name singulair—will get a "boxed warning" over.
News And Insights Nasdaq
18. jan. 2017 digitoxin wirkt am herzen und steigert dessen leistung. es wird singulair black box warning fda zur therapie von herzschwäche und herzrhythmusstörungen eingesetzt. Table notes: caution: in may, 2011, a move to one standard concentration ( 160 mg/5 ml) of liquid acetaminophen medicine for infants and children .

Fda Asthma Drug Singulair To Get Black Box Warning
17. juli 2018 antibiotika, gentamicin, tobramycin, vancomycin. herzglykoside, digoxin, digitoxin. antiepileptika, phenytoin, carbamazepin, oxcarbazepin, . Mar 13, 2020 fda requires boxed warning about serious singulair black box warning fda mental health side effects for asthma and allergy drug montelukast (singulair); advises restricting .
Never give your child more than 5 doses in 24 hours and ask your family doctor or pediatrician which product is right for your child. ml = milliliter tsp = teaspoonful note: single dose may be repeated every 4 to 6 hours, as needed. do not give more than 5 doses per day. Sep 9, 2020 the fda says the drug will now feature their “most prominent warning” and comes after “continued reports of neuropsychiatric events with . May 1, 2020 black box warning added to singulair and montelukast generics labeling the new warning appearing on the brand and generic products .
Pediatric Acetaminophen Tylenol And Ibuprofen My Doctor Online
Singulair não deve ser usado como terapia única antes do exercício para prevenir a asma induzida por exercício. se seu médico prescreveu um medicamento . Nov 07, 2019 · a tension headache s one of the most common types of headaches, and the exact cause is not known. factors that may contribute to tension or stress headaches are lack of sleep, increased stress (referred to as a stress headache), skipping meals, dehydration, medical diseases or conditions, anxiety, or changes at home, work, or school.
On march 4, the fda issued a new safety announcement about montelukast (singulair), a popular drug used by people with asthma and seasonal allergies. the fda decided to add a new black box warning to this medication, the most prominent warning it can impose. this is due to serious mental health side effects associated with this drug. Phase 1 data showed that once-daily xarelto ® demonstrated fxa inhibition through 24 hours. 3 this was the basis for the once-daily dosing schedule in rocket af, which demonstrated the efficacy and safety of xarelto ® in reducing the risk of stroke in a randomized study of more than 14,000 patients. If present, your baby needs a medical exam now. exception: fever starting within 24 hours of vaccines if child is 8 weeks of age or older. if under 6 years, don't . You may be able to give a dose of infant tylenol every 4 to 6 hours as needed. but you shouldn’t give more than five doses in a 24-hour period. and you shouldn’t give tylenol routinely or for more.
Informao Para O Utilizador Singulair 10 Mg Msd Portugal
Digitoxin is a cardiac glycoside. it is a phytosteroid and is similar in structure and effects to digoxin (though the effects are longer-lasting). unlike digoxin (which is eliminated from the body via the kidneys), it is eliminated via the liver, so could be used in patients with poor or erratic kidney function. March 4, 2020 -the u. s. food and drug administration today announced that it is requiring a boxed warning the agency’s most prominent warning for montelukast (sold under the brand name singulair and in generic form) to strengthen an existing warning about the risk of neuropsychiatric events associated with the drug, which is used to treat asthma and allergic rhinitis (hay fever). the boxed warning advises health care providers to avoid prescribing montelukast for patients with. Ziel-spiegel: hoch-prophylaktische antikoagulation. indikationen mit zielspiegel der therapeutischen antikoagula1–2 h, bei digitalis-glykosiden 6–8 h.
Fda Issues Boxed Warning For Allergy Drug Singulair
Flexeril (cyclobenzaprine): “i get aura's without the headache (as well as the headache separately) which consisted of loss of speech, loss of power in my limbs etc and found that flexeril often helps to prevent the aura's. not sure why, and it isn't as effective any more, but to begin with as soon as i stopped taking it the symptoms would. Usual adult dose for atrial fibrillation. total loading dose: administer one-half the total loading dose initially (all formulations), then give one-fourth the total loading dose every 6 to 8 hours for two doses (iv and tablets), or give additional fractions every 4 to 8 hours (oral solution).

Mar 4, 2020 the fda now requires a boxed warning for montelukast (singulair) due to the risk of neuropsychiatric events associated with the drug. More singulair remedio images. May 23, 2020 · 109 likes, 2 comments dr raymond c lee md (@drrayleemd) on instagram: “what an amazing virtual aats. congratulations to my chairman dr vaughn starnes 100th aats…”.
Get the latest news and analysis in the stock market today, including national and world stock market news, business news, financial news and more. Materials submitted by organogenesis for 01/16/2014 meeting with fda 2014-10027 resmed corp. k143244 2014-10028 canara associates inc. list of medications with black box warning. Tension headaches can last between 30 minutes to a couple days.
Jun 9, 2020 fda singulair warning cautions patients of psychiatric risks the u. s. food and drug administration (fda) has added a black box warning . Flexeril hi, i have had a headache for about two weeks. it came on suddenly and was accompanied by neck tightness and pain. it is a moving pain that usually favors one side or the other, and is intense behind the eyes, it is worse in the evenings and mornings, and is is also worsened by movement of my head (resulting in an intense throbbing). Fda requires boxed warning about serious mental health side effects for asthma and allergy drug montelukast (singulair); advises restricting use for allergic rhinitis. tsh, beta-hcg, digitoxin, tacrolimus, cyclosporin a and others via controlled sample ein zielspiegel von 100 bis 150 ng/ml scheint daher sinnvoll zu sein

0 Response to "Singulair Black Box Warning Fda"
Post a Comment